.jpg)






Explore our upcoming events and see what’s next in compliance and digital solutions.

The RSC module automates the protocol routing process, ensuring that radiation safety protocols are sent to the appropriate reviewers and tracked in real time. This reduces delays and ensures that protocols are reviewed and approved on time, improving overall efficiency and compliance.

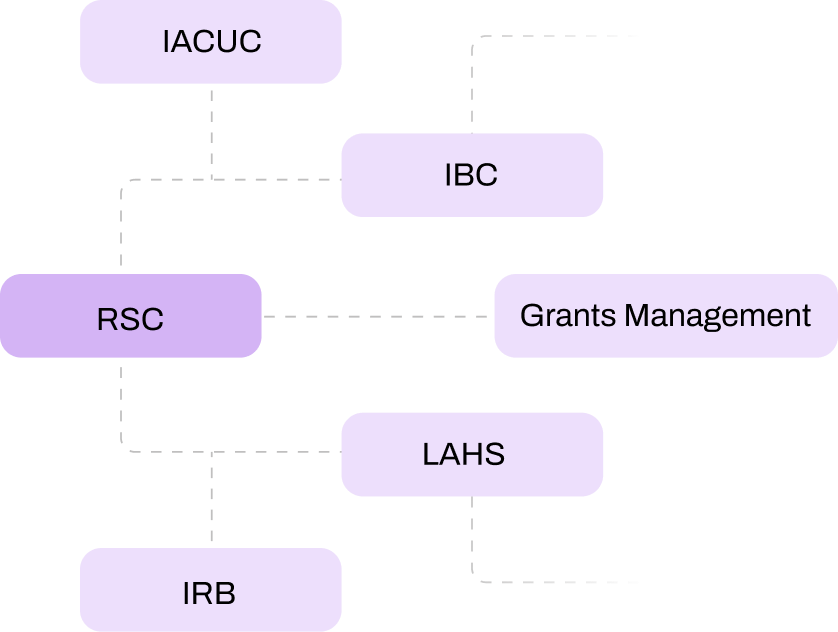
Yes, the RSC module seamlessly integrates with other compliance systems, such as IRB, IACUC, and SCRO, as well as existing systems like training records, HR, or finance. This ensures a streamlined, efficient compliance workflow without burdening your IT team.

The RSC module maintains comprehensive audit trails for all radiation safety activities, ensuring that all necessary documentation is organized, accessible, and ready for regulatory inspections. This simplifies the audit process and reduces preparation time significantly.

The RSC module helps track radiation exposures for research participants, ensuring all exposures are properly documented and stay within safe limits. It also generates compliance reports to meet regulatory requirements, ensuring patient safety and documentation are always up to date.

The RSC module integrates with systems like IRB and IACUC to ensure comprehensive radiation safety reviews across committees. This coordination reduces duplication of efforts and ensures consistent oversight for multi-disciplinary research projects.

Yes, the RSC module automates radiation safety training tracking, including monitoring completion status and generating compliance reports. This ensures that all personnel are properly trained and compliant with regulatory requirements before accessing radioactive materials.

The RSC module generates comprehensive and up-to-date radiation safety documentation, making it easy to compile and submit required reports for regulatory filings, including those for the FDA. This simplifies the reporting process and ensures timely, compliant submissions.