.jpg)






Explore our upcoming events and see what’s next in compliance and digital solutions.

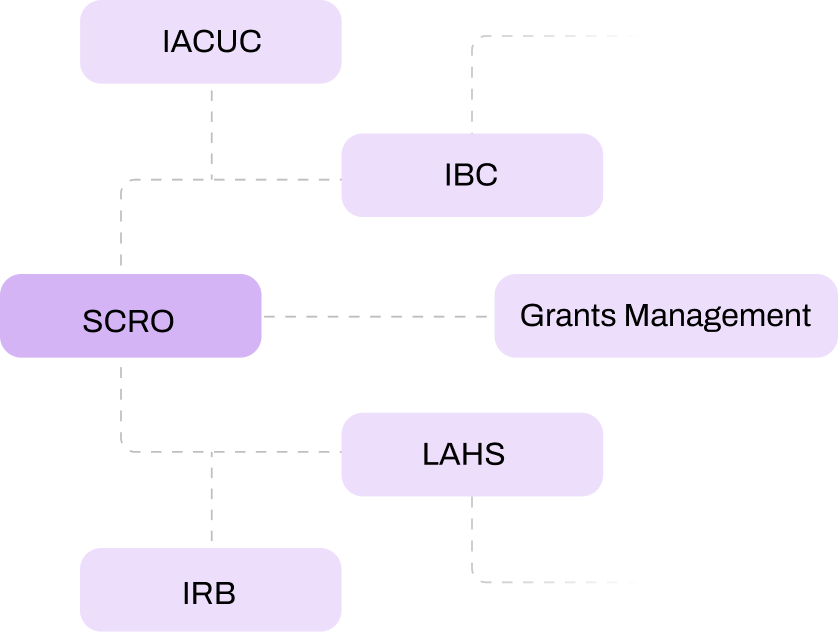
SCRO simplifies the protocol approval process by automating routing to the right reviewers, ensuring faster approvals and eliminating delays. The system provides real-time tracking, allowing you to monitor the status of every protocol from submission to approval. Automated reminders for renewals and amendments ensure compliance deadlines are always met, so you can keep research moving forward without bottlenecks.

If you already have a protocol management system, integrating SCRO will streamline and enhance your existing processes. Our platform adds automated workflows, real-time tracking, and centralized access to all protocol data, improving efficiency and reducing administrative load. You’ll still benefit from the SCRO system’s added features without replacing your current setup.

SCRO eProtocol module automates key processes such as protocol submission, routing, approvals, and tracking, eliminating manual steps and reducing delays. Real-time dashboards keep everyone on the same page, while automated reminders ensure timely renewals and amendments. This efficiency frees up your team to focus more on research rather than administrative tasks.

Yes, SCRO module is built to ensure compliance with all relevant regulatory standards, including AAHRPP, FDA, EMA, and ICH requirements. It tracks every action in the protocol process with a complete audit trail, ensuring full transparency. Whether it's for internal reviews or federal inspections, you'll be fully prepared with audit-ready documentation.

Absolutely. SCRO is highly customizable. Whether you’re in academia, biotech, pharma, or any other sector, our system adapts to your unique workflows. You can set up automated routing, approval processes, and compliance tracking to match your exact requirements, ensuring a seamless fit into your organization’s operations.

Our dedicated support team is available to assist you with any technical difficulties. We offer training resources, live support, and troubleshooting to ensure you get the most out of the SCRO module. Additionally, the system is user-friendly and designed to be intuitive, reducing the need for ongoing technical intervention.

Implementation times can vary depending on your organization's size and existing systems, but most users can expect to see SCRO fully integrated and operational within a few weeks. Our team will guide you through the setup process, ensuring a smooth transition without disrupting your research activities. Plus, with its easy integration capabilities, your team will be able to start using it quickly.